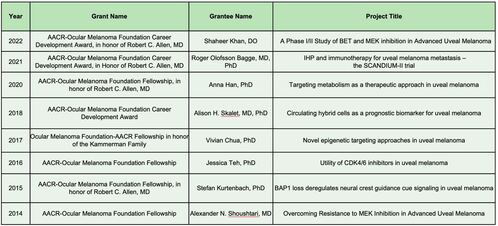
OMF Funded Research
Investing in promising cancer research is the primary path to “See a Cure” and since its founding in 2003, OMF has raised nearly $3 million to fund cancer research in addition to patient education and direct patient/caregiver support through our TAG and PAP programs.
Since 2013, OMF has partnered with the American Association of Cancer Research (AACR) to provide tailored research grants such as Career Development Awards and annual, peer-reviewed research awards to postdoctoral or clinical research fellows who are conducting ocular melanoma research. OMF also gives directly to leading institutions such as Thomas Jefferson University through their MRIE program established by Takami Sato.
These grants not only provide funding for ocular melanoma research, they also encourage both promising and established researchers to establish and continue successful careers in ophthalmology, ocular oncology, melanoma biology, or a similar field.
Since 2013, OMF has partnered with the American Association of Cancer Research (AACR) to provide tailored research grants such as Career Development Awards and annual, peer-reviewed research awards to postdoctoral or clinical research fellows who are conducting ocular melanoma research. OMF also gives directly to leading institutions such as Thomas Jefferson University through their MRIE program established by Takami Sato.
These grants not only provide funding for ocular melanoma research, they also encourage both promising and established researchers to establish and continue successful careers in ophthalmology, ocular oncology, melanoma biology, or a similar field.
AACR Partnership Grants
2022 AACR-OMF Career Development Award - $150,000


Research Overview
Uveal melanoma is a rare melanoma subtype associated with poor outcomes in the metastatic setting. Virtually all cases harbor activating mutations of GNAQ, GNA11, PLCB4 or CYSLTR2 which result in activation of the MAP kinase pathway, amongst others. In addition, epigenetic dysregulation is known to play a critical role in disease pathogenesis. Based on preclinical data showing that the combination of MEK inhibition and Bromodomain and Extra-terminal (BET) protein inhibition (an epigenetic regulator) is associated with synergistic anti-tumor activity, Dr. Khan will investigate the safety and efficacy of combined BET and MEK inhibition in a multi-center phase I/II clinical trial in patients with metastatic uveal melanoma. Translational components of this study will assess potential mechanisms of treatment response and resistance using tumor and blood samples from patients.
Biography
Dr. Khan is an Assistant Professor of Medicine at the Columbia University Herbert Irving Comprehensive Cancer Center. He received his medical degree from the New York College of Osteopathic Medicine after which he completed his training in internal medicine at Medstar Georgetown University Hospital and hematology and medical oncology at Columbia University Medical Center. Dr. Khan’s research is focused on the development of novel therapies for the treatment of cutaneous malignancies, including cutaneous squamous cell carcinoma and uveal melanoma.
Acknowledgment of Support
It is a tremendous honor to be the recipient of the 2022 AACR-Ocular Melanoma Foundation Career Development Award, in honor of Robert C. Allen, MD. This award will be critical as I develop my research career and will be an important resource to facilitate the conduct and analysis of this promising clinical trial.
Uveal melanoma is a rare melanoma subtype associated with poor outcomes in the metastatic setting. Virtually all cases harbor activating mutations of GNAQ, GNA11, PLCB4 or CYSLTR2 which result in activation of the MAP kinase pathway, amongst others. In addition, epigenetic dysregulation is known to play a critical role in disease pathogenesis. Based on preclinical data showing that the combination of MEK inhibition and Bromodomain and Extra-terminal (BET) protein inhibition (an epigenetic regulator) is associated with synergistic anti-tumor activity, Dr. Khan will investigate the safety and efficacy of combined BET and MEK inhibition in a multi-center phase I/II clinical trial in patients with metastatic uveal melanoma. Translational components of this study will assess potential mechanisms of treatment response and resistance using tumor and blood samples from patients.
Biography
Dr. Khan is an Assistant Professor of Medicine at the Columbia University Herbert Irving Comprehensive Cancer Center. He received his medical degree from the New York College of Osteopathic Medicine after which he completed his training in internal medicine at Medstar Georgetown University Hospital and hematology and medical oncology at Columbia University Medical Center. Dr. Khan’s research is focused on the development of novel therapies for the treatment of cutaneous malignancies, including cutaneous squamous cell carcinoma and uveal melanoma.
Acknowledgment of Support
It is a tremendous honor to be the recipient of the 2022 AACR-Ocular Melanoma Foundation Career Development Award, in honor of Robert C. Allen, MD. This award will be critical as I develop my research career and will be an important resource to facilitate the conduct and analysis of this promising clinical trial.
2021 AACR-OMF Career Development Award - $150,000

Research Overview
Due to the failure of current treatment regimens to significantly prolong the survival of patients with uveal melanoma metastases, it is of outmost importance to develop new treatments for this patient group. Dr. Bagge is set to conduct a Phase I clinical trial on the combination of isolated hepatic perfusion with melphalan and the immune checkpoint inhibitors ipilimumab and nivolumab in uveal melanoma patients with liver metastasis. To further delineate the immunological effects of the combined treatment he plans to conduct advanced FACS and genomic analyses of tumour and blood samples from enrolled patients.
Biography
Dr. Bagge is a senior consultant surgeon at Sahlgrenska University Hospital and an associate professor at the University of Gothenburg, Sweden. He is responsible for isolated hyperthermic perfusion in Sweden and treats all patients requiring either isolated limb perfusion or isolated hepatic perfusion. Dr. Bagge is also a research group leader at the Wallenberg Center for Molecular and Translational Medicine. He conducts both clinical and preclinical research, with a special focus on treatments for both cutaneous and ocular melanoma.
Acknowledgment of Support
I am truly honored and grateful to be the recipient of the 2021 AACR-Ocular Melanoma Foundation Career Development Award, in honor of Robert C. Allen, MD. This recognition is extremely important in our joint quest to address the unmet need for effective treatments for patients with metastatic ocular melanoma.
Due to the failure of current treatment regimens to significantly prolong the survival of patients with uveal melanoma metastases, it is of outmost importance to develop new treatments for this patient group. Dr. Bagge is set to conduct a Phase I clinical trial on the combination of isolated hepatic perfusion with melphalan and the immune checkpoint inhibitors ipilimumab and nivolumab in uveal melanoma patients with liver metastasis. To further delineate the immunological effects of the combined treatment he plans to conduct advanced FACS and genomic analyses of tumour and blood samples from enrolled patients.
Biography
Dr. Bagge is a senior consultant surgeon at Sahlgrenska University Hospital and an associate professor at the University of Gothenburg, Sweden. He is responsible for isolated hyperthermic perfusion in Sweden and treats all patients requiring either isolated limb perfusion or isolated hepatic perfusion. Dr. Bagge is also a research group leader at the Wallenberg Center for Molecular and Translational Medicine. He conducts both clinical and preclinical research, with a special focus on treatments for both cutaneous and ocular melanoma.
Acknowledgment of Support
I am truly honored and grateful to be the recipient of the 2021 AACR-Ocular Melanoma Foundation Career Development Award, in honor of Robert C. Allen, MD. This recognition is extremely important in our joint quest to address the unmet need for effective treatments for patients with metastatic ocular melanoma.
2020 AACR-Ocular Melanoma Foundation Fellowship - $50,000
Awarded to: Anna Han, PhD, Thomas Jefferson University
Research Overview
Dr. Han's research proposal includes two aims: (1) to define unique metabolic features of UM that can be targetable, and (2) to identify metabolic functions of BAP1 mutations in UM. Dr. Han will study the possible roles of GNAQ/GNA11 mutations along with other frequently dysregulated oncogenic signaling pathways such as the PI3K/Akt/mTOR pathway, in upregulating lipogenesis in UM, and also compare BAP1-deficient and BAP1 re-expressed UM cell lines previously generated, eliminating confounding factors due to cell type differences.
Read more including the full abstract here.
Biography
Anna Han is a Post Doctoral Fellow at Thomas Jefferson University and a member of the Uveal Melanoma Working Group. Anna studies the therapeutic options that target metabolism in uveal melanoma and in subtypes of BAP1-deficient uveal melanoma.
Research Overview
Dr. Han's research proposal includes two aims: (1) to define unique metabolic features of UM that can be targetable, and (2) to identify metabolic functions of BAP1 mutations in UM. Dr. Han will study the possible roles of GNAQ/GNA11 mutations along with other frequently dysregulated oncogenic signaling pathways such as the PI3K/Akt/mTOR pathway, in upregulating lipogenesis in UM, and also compare BAP1-deficient and BAP1 re-expressed UM cell lines previously generated, eliminating confounding factors due to cell type differences.
Read more including the full abstract here.
Biography
Anna Han is a Post Doctoral Fellow at Thomas Jefferson University and a member of the Uveal Melanoma Working Group. Anna studies the therapeutic options that target metabolism in uveal melanoma and in subtypes of BAP1-deficient uveal melanoma.
2018 AACR-OMF Career Development Award - $150,000

Research Overview
Uveal melanoma is an aggressive intraocular cancer associated with high rates of metastatic disease. Although highly predictive molecular prognostic testing for uveal melanoma is available, it requires tumor tissue that is not always accessible via tumor biopsy. Dr. Skalet’s group has identified circulating cells in patients with uveal melanoma that are hybrids of macrophage and tumor cells. She is set to determine the prognostic power of these circulating hybrid cells in uveal melanoma patients undergoing primary treatment and to correlate the levels of these cells with clinical staging and gene expression profile classification.
Biography
Dr. Skalet completed her MD/PhD at the University of Pennsylvania. Following an internship at Children’s Hospital of Philadelphia, she completed her ophthalmology residency at the University of California, San Francisco, and subspecialty training in ocular oncology and ophthalmic pathology at Oregon Health and Science University (OHSU). She is currently an assistant professor of ophthalmology at the Casey Eye Institute, OHSU. Her research focuses on early detection of uveal melanoma and vision-threatening complications of radiation treatment.
Acknowledgement of Support
This grant award supports exploration of a novel circulating tumor cell population as a non-invasive prognostic biomarker and source for genetic information in uveal melanoma. Development of this novel biomarker permits pursuit of a line of inquiry with potential to open a new conceptual area in uveal melanoma research.
Uveal melanoma is an aggressive intraocular cancer associated with high rates of metastatic disease. Although highly predictive molecular prognostic testing for uveal melanoma is available, it requires tumor tissue that is not always accessible via tumor biopsy. Dr. Skalet’s group has identified circulating cells in patients with uveal melanoma that are hybrids of macrophage and tumor cells. She is set to determine the prognostic power of these circulating hybrid cells in uveal melanoma patients undergoing primary treatment and to correlate the levels of these cells with clinical staging and gene expression profile classification.
Biography
Dr. Skalet completed her MD/PhD at the University of Pennsylvania. Following an internship at Children’s Hospital of Philadelphia, she completed her ophthalmology residency at the University of California, San Francisco, and subspecialty training in ocular oncology and ophthalmic pathology at Oregon Health and Science University (OHSU). She is currently an assistant professor of ophthalmology at the Casey Eye Institute, OHSU. Her research focuses on early detection of uveal melanoma and vision-threatening complications of radiation treatment.
Acknowledgement of Support
This grant award supports exploration of a novel circulating tumor cell population as a non-invasive prognostic biomarker and source for genetic information in uveal melanoma. Development of this novel biomarker permits pursuit of a line of inquiry with potential to open a new conceptual area in uveal melanoma research.
2017 AACR-OMF/Kammerman Family Fellowship - $50,000
Awarded to: Vivian Chua, PhD, Sidney Kimmel Cancer Center, Thomas Jefferson University (Philadelphia, PA)
Novel Epigenetic Targeting Approaches in Uveal Melanoma (UM)
Dr. Chua's reseach focuses on how bromodomain protein inhibitors can effectively reduce metastatic UM cell growth. A second aim of the research is to investigate whether cells in the liver microenvironment would produce factors/proteins that may alter the responses of UM tumor cells to bromodomain inhibitors.
Novel Epigenetic Targeting Approaches in Uveal Melanoma (UM)
Dr. Chua's reseach focuses on how bromodomain protein inhibitors can effectively reduce metastatic UM cell growth. A second aim of the research is to investigate whether cells in the liver microenvironment would produce factors/proteins that may alter the responses of UM tumor cells to bromodomain inhibitors.
2016 AACR-Ocular Melanoma Foundation Fellowship - $50,000
Jessica Teh, PhD, Sidney Kimmel Cancer Center, Thomas Jefferson University (Philadelphia, PA)
Title: Utility of CDK4/6 Inhibitors in Uveal Melanoma
After treatment of primary uveal melanoma tumors, 50% of patients will develop macro-metastases and currently there are no FDA-approved targeted inhibitor treatments for metastatic UM. MEK-ERK1/2 signaling is activated frequently in uveal melanoma due to driver mutations in either GNAQ or GNA11. While MEK inhibitors are FDA-approved in cutaneous melanoma, they provide a 14% response rate and modestly improve progression-free survival in uveal melanoma. Dr. Teh’s proposal aims to utilize an in vivo reporter model to monitor the effects of the combination of MEK and CDK4/6 inhibitors and to identify optimal dosing schemes for this combination. Additionally, she will work on determining the effect of BAP1 status in the modulation of response to the combination leveraging new metastatic uveal melanoma cell lines, a new in vivo metastatic colonization model, and uveal melanoma patient samples from clinical trials. Dr. Teh hopes to ultimately provide the pre-clinical basis for targeted inhibitor combinations in late-stage uveal melanoma.
Title: Utility of CDK4/6 Inhibitors in Uveal Melanoma
After treatment of primary uveal melanoma tumors, 50% of patients will develop macro-metastases and currently there are no FDA-approved targeted inhibitor treatments for metastatic UM. MEK-ERK1/2 signaling is activated frequently in uveal melanoma due to driver mutations in either GNAQ or GNA11. While MEK inhibitors are FDA-approved in cutaneous melanoma, they provide a 14% response rate and modestly improve progression-free survival in uveal melanoma. Dr. Teh’s proposal aims to utilize an in vivo reporter model to monitor the effects of the combination of MEK and CDK4/6 inhibitors and to identify optimal dosing schemes for this combination. Additionally, she will work on determining the effect of BAP1 status in the modulation of response to the combination leveraging new metastatic uveal melanoma cell lines, a new in vivo metastatic colonization model, and uveal melanoma patient samples from clinical trials. Dr. Teh hopes to ultimately provide the pre-clinical basis for targeted inhibitor combinations in late-stage uveal melanoma.
2015 AACR-Ocular Melanoma Foundation Fellowship - $50,000

Stefan Kurtenbach, MD, Miller School of Medicine of the University of Miami (Miami, FL)
Title: BAP1 Loss Deregulates Neural Crest Guidance Cue Signaling in Uveal Melanoma
Findings: Although Dr. Kurtenbach’s research is ongoing, initial results found PRAME mRNA as the most significant predictor for metastasis in Class 1 tumors. These results have been published in “Clinical Cancer Research” (http://clincancerres.aacrjournals.org/content/22/5/1234).
Title: BAP1 Loss Deregulates Neural Crest Guidance Cue Signaling in Uveal Melanoma
Findings: Although Dr. Kurtenbach’s research is ongoing, initial results found PRAME mRNA as the most significant predictor for metastasis in Class 1 tumors. These results have been published in “Clinical Cancer Research” (http://clincancerres.aacrjournals.org/content/22/5/1234).
2014 AACR-Ocular Melanoma Foundation Fellowship - $50,000

Alexander Shoushtari, MD, Sloan-Kettering Institute for Cancer Research (New York, NY)
Title: Overcoming Resistance to MEK Inhibition in Advanced Uveal Melanoma
Synopsis: Dr. Shoushtari helped coordinate a randomized, multicenter trial combining a MEK inhibitor called trametinib with/without an AKT inhibitor called GSK2141795. This represented a novel approach to treating ocular melanoma and tested the scientific hypothesis that combined MEK and AKT inhibition is better than MEK inhibition alone.
As part of the clinical trial, tumor biopsies were taken before and during treatment. Dr. Shoushtari and colleagues analyzed the genetic changes during treatment in the biopsy specimens and compared patients whose tumors responded to therapy with those whose tumors did not respond to therapy. Comparing these tumors will help shed light on how uveal melanomas rely on MAPK, AKT, or other growth signaling pathways to grow. The results of the research are being applied to help plan new approaches to treating uveal melanoma, and will be generalizable to other types of tumors that rely on these growth pathways.
Findings: Two benefits of the study were the amassing of the largest annotated biorepository of uveal melanoma and cultivating the research across multiple centers in the United States and Europe. In Shoushtari’s words, “The fact that we were able to accrue rapidly across multiple centers shows unequivocally that multicenter trials in this rare disease can be conducted efficiently.”
Title: Overcoming Resistance to MEK Inhibition in Advanced Uveal Melanoma
Synopsis: Dr. Shoushtari helped coordinate a randomized, multicenter trial combining a MEK inhibitor called trametinib with/without an AKT inhibitor called GSK2141795. This represented a novel approach to treating ocular melanoma and tested the scientific hypothesis that combined MEK and AKT inhibition is better than MEK inhibition alone.
As part of the clinical trial, tumor biopsies were taken before and during treatment. Dr. Shoushtari and colleagues analyzed the genetic changes during treatment in the biopsy specimens and compared patients whose tumors responded to therapy with those whose tumors did not respond to therapy. Comparing these tumors will help shed light on how uveal melanomas rely on MAPK, AKT, or other growth signaling pathways to grow. The results of the research are being applied to help plan new approaches to treating uveal melanoma, and will be generalizable to other types of tumors that rely on these growth pathways.
Findings: Two benefits of the study were the amassing of the largest annotated biorepository of uveal melanoma and cultivating the research across multiple centers in the United States and Europe. In Shoushtari’s words, “The fact that we were able to accrue rapidly across multiple centers shows unequivocally that multicenter trials in this rare disease can be conducted efficiently.”
Thomas Jefferson University MRIE Awards
2023 Ocular Melanoma Foundation Pilot Awardee #1
Dr. Mizue Terai: “Generation and Optimization of Humanized Mice to Investigate Metastatic Uveal Melanoma Immunity”
Lay Abstract:
Melanoma of the eye, also known as uveal melanoma (UM), is a rare tumor that originates from pigment- producing cells (melanocytes) in the eye. Although rare compared to cutaneous (skin) melanoma, it is the most common primary intraocular malignancy in adults. During the past decade, substantial progress has been achieved in the treatment of primary eye lesions while preserving the affected eye. Nevertheless, up to 50% of UM patients subsequently develop systemic spread (metastasis) after successful therapy of the primary tumor in the eye. About 80-90% of these metastases develop in the liver and patients often die of liver failure. Once UM cells escape from the eye and spread to the liver where tumors rapidly progress, patients die within 4 to 15 months. Metastatic UM (MUM) is highly resistant to chemotherapy and inhibitors. No significant survival improvement has been made with various these therapies.
In the past decade, immunotherapy, a therapy in which the patient’s own immune system is activated to fight against cancer cells, has become a powerful approach to treat metastatic skin melanoma. Several immunotherapies called immune check point inhibitors, including ipilimumab, nivolumab, pembrolizumab, and relatlimab, were successfully tested in patients with metastatic skin melanoma and they were approved by the Food and Drug Administration (FDA) for treatment. These medications improve overall survival of patients with skin melanoma and become a cure for patients. Unfortunately, these medicaines do not work well for MUM and only a limited number of UM patients receive clinical benefit from them. Recently a new immunotherapy medicine, tebentafusp (Kimmtrak®), was approved by the FDA for the treatment of MUM patients. Although survival was prolonged for approximately 6 months with this new immunotherapy compared to standard care treatments, dramatic shrinkage of metastasis was rare. Less than 10% of patients showed shrinkage of metastasis by follow-ups. Therefore, there is still an unmet need for better treatment for MUM patients.
Based on our and other researchers' data, we would like to further explore the reasons for the low responsiveness of MUM to immunotherapy. Unlike cutaneous melanoma, which develops spontaneously in normal mice with immune systems, there are no animal models with immune systems to study UM. Therefore, not enough research has been done to confirm our speculation. We have already developed an animal model of liver metastasis of UM that does not have an immune system. Our approach is to establish a mouse model with human immune cells (humanized mice). Using this model, we can study how tumors manipulate their microenvironment, how tumors enhance the anti-cancer drug resistance response, and how we induce the efficacy of immunotherapeutics against human MUM.
To achieve these goals, we proposed the following two projects in this pilot award application:
Aim 1: Generate humanized mouse model. Our primary aim is to develop a mouse model with human immune cells after transplantation of human hematopoietic stem cells. Individual donors may differ in age and/or sex. At least three donors will be employed to investigate the induction of immune cells and their functional assay.
a. Characterization of Hematopoietic Stem Cells generated from humanized mouse.
b. Test immune potency of the engrafted human immune cells.
c. Evaluation of the longevity of the humanized mice with the possibility of attack to their own tissues after human hematopoietic stem cells transplantation.
Aim 2: Investigate humanized immune system inside a mouse with human MUM. This aim is to test the severity of immune attack to MUM after the transplant of the human hematopoietic stem cells into the mice to evaluate the efficacy of immunotherapy agents.
a. Evaluation of tolerability and growth of the MUM cells in subcutaneous area and in the liver from humanized mice.
b. Evaluation of immune activities in humanized mice with MUM cells.
Our long-term goals are to identify pharmacological approaches and to investigate effective immunotherapeutic drugs or combinational approaches with inhibitor(s) or chemotherapeutic drug(s) to achieve effective killing of MUM cells.
Melanoma of the eye, also known as uveal melanoma (UM), is a rare tumor that originates from pigment- producing cells (melanocytes) in the eye. Although rare compared to cutaneous (skin) melanoma, it is the most common primary intraocular malignancy in adults. During the past decade, substantial progress has been achieved in the treatment of primary eye lesions while preserving the affected eye. Nevertheless, up to 50% of UM patients subsequently develop systemic spread (metastasis) after successful therapy of the primary tumor in the eye. About 80-90% of these metastases develop in the liver and patients often die of liver failure. Once UM cells escape from the eye and spread to the liver where tumors rapidly progress, patients die within 4 to 15 months. Metastatic UM (MUM) is highly resistant to chemotherapy and inhibitors. No significant survival improvement has been made with various these therapies.
In the past decade, immunotherapy, a therapy in which the patient’s own immune system is activated to fight against cancer cells, has become a powerful approach to treat metastatic skin melanoma. Several immunotherapies called immune check point inhibitors, including ipilimumab, nivolumab, pembrolizumab, and relatlimab, were successfully tested in patients with metastatic skin melanoma and they were approved by the Food and Drug Administration (FDA) for treatment. These medications improve overall survival of patients with skin melanoma and become a cure for patients. Unfortunately, these medicaines do not work well for MUM and only a limited number of UM patients receive clinical benefit from them. Recently a new immunotherapy medicine, tebentafusp (Kimmtrak®), was approved by the FDA for the treatment of MUM patients. Although survival was prolonged for approximately 6 months with this new immunotherapy compared to standard care treatments, dramatic shrinkage of metastasis was rare. Less than 10% of patients showed shrinkage of metastasis by follow-ups. Therefore, there is still an unmet need for better treatment for MUM patients.
Based on our and other researchers' data, we would like to further explore the reasons for the low responsiveness of MUM to immunotherapy. Unlike cutaneous melanoma, which develops spontaneously in normal mice with immune systems, there are no animal models with immune systems to study UM. Therefore, not enough research has been done to confirm our speculation. We have already developed an animal model of liver metastasis of UM that does not have an immune system. Our approach is to establish a mouse model with human immune cells (humanized mice). Using this model, we can study how tumors manipulate their microenvironment, how tumors enhance the anti-cancer drug resistance response, and how we induce the efficacy of immunotherapeutics against human MUM.
To achieve these goals, we proposed the following two projects in this pilot award application:
Aim 1: Generate humanized mouse model. Our primary aim is to develop a mouse model with human immune cells after transplantation of human hematopoietic stem cells. Individual donors may differ in age and/or sex. At least three donors will be employed to investigate the induction of immune cells and their functional assay.
a. Characterization of Hematopoietic Stem Cells generated from humanized mouse.
b. Test immune potency of the engrafted human immune cells.
c. Evaluation of the longevity of the humanized mice with the possibility of attack to their own tissues after human hematopoietic stem cells transplantation.
Aim 2: Investigate humanized immune system inside a mouse with human MUM. This aim is to test the severity of immune attack to MUM after the transplant of the human hematopoietic stem cells into the mice to evaluate the efficacy of immunotherapy agents.
a. Evaluation of tolerability and growth of the MUM cells in subcutaneous area and in the liver from humanized mice.
b. Evaluation of immune activities in humanized mice with MUM cells.
Our long-term goals are to identify pharmacological approaches and to investigate effective immunotherapeutic drugs or combinational approaches with inhibitor(s) or chemotherapeutic drug(s) to achieve effective killing of MUM cells.
2023 Ocular Melanoma Foundation Pilot Awardee #2
Dr. Vivian Chua: “Targeting Metabolism to Induce Gasdermin-Mediated Inflammatory Cell Death in Uveal Melanoma”
Lay Abstract:
Advanced stage uveal melanoma often responds poorly to a number of therapeutic options including immunotherapy which helps the immune system to fight cancer. A type of immunotherapy called immune checkpoint inhibitors successfully treat a subset of patients with skin melanoma but patients with uveal melanoma respond poorly to these drugs in clinical trials. We predict that there is a lack of recognition of uveal melanoma tumors by the immune system. Our studies in skin melanoma found that a process that cancer cells undergo called pyroptosis, a type of inflammatory cell death, releases proteins from cancer cells that can attract the immune system to the cancer cells. A critical protein involved in pyroptosis called gasdermins are present in uveal melanoma. One way to enhance pyroptosis and activate the gasdermin proteins is by metabolic inhibitors; some of which have been approved by the U.S. Food and Drug Administration (FDA) for the treatment of other diseases. We have data showing that a metabolic inhibitor causes pyroptosis in uveal melanoma cells. These cells were isolated from patient's tumor, grown as cultures, and should retain the majority of the tumor's characteristics. In this proposal, we will identify how metabolic inhibitors induce pyroptosis and which classes of metabolic inhibitors are capable of increasing pyroptosis. Importantly, we will determine whether metabolic inhibitors can enhance the efficacy of immune checkpoint therapy in uveal melanoma. Our findings will show a treatment strategy that potentially improves patient survival.
Advanced stage uveal melanoma often responds poorly to a number of therapeutic options including immunotherapy which helps the immune system to fight cancer. A type of immunotherapy called immune checkpoint inhibitors successfully treat a subset of patients with skin melanoma but patients with uveal melanoma respond poorly to these drugs in clinical trials. We predict that there is a lack of recognition of uveal melanoma tumors by the immune system. Our studies in skin melanoma found that a process that cancer cells undergo called pyroptosis, a type of inflammatory cell death, releases proteins from cancer cells that can attract the immune system to the cancer cells. A critical protein involved in pyroptosis called gasdermins are present in uveal melanoma. One way to enhance pyroptosis and activate the gasdermin proteins is by metabolic inhibitors; some of which have been approved by the U.S. Food and Drug Administration (FDA) for the treatment of other diseases. We have data showing that a metabolic inhibitor causes pyroptosis in uveal melanoma cells. These cells were isolated from patient's tumor, grown as cultures, and should retain the majority of the tumor's characteristics. In this proposal, we will identify how metabolic inhibitors induce pyroptosis and which classes of metabolic inhibitors are capable of increasing pyroptosis. Importantly, we will determine whether metabolic inhibitors can enhance the efficacy of immune checkpoint therapy in uveal melanoma. Our findings will show a treatment strategy that potentially improves patient survival.
2023 Ocular Melanoma Foundation Team Awardee
Dr. Isidore Rigoutsos: “Short Non-coding RNAs as Biomarkers for Response to Immunotherapy in Metastatic Uveal Melanoma”
Lay Abstract
Melanoma of the eye, also known as uveal melanoma (UM), is a rare tumor that originates from pigment producing cells (melanocytes) in the eye. Compared to cutaneous (skin) melanoma UM is rare, yet it is the most common primary intraocular malignancy in adults. During the past decade, substantial progress has been achieved in the treatment of primary eye lesions that allow for the preservation of the affected eye. However, nearly one half of UM patients progress to develop metastases that most often localize to the liver. Rapid tumor progression in the liver leads to death within 4 to 15 months. The use of chemotherapy has proven of little value to date because metastatic UM (MUM) is highly resistant to it. Consequently, during the last decade, researchers have been investigating an alternative form of therapy called immunotherapy.
Immunotherapy works differently than chemotherapy. Here, the patient’s immune system is activated in a targeted manner to attack cancer cells. Immunotherapy has proven very powerful in numerous contexts and several drugs (“immune check point inhibitors”) have already been approved by the US Food and Drug Administration. Immune check point inhibitors greatly improved the overall survival of skin melanoma patients but have shown little benefit for UM patients. However, a new FDA-approved therapy for UM, Tebentafusp-tebn, is more promising. The therapy works by increasing the numbers of cancer-fighting cells and is currently the preferred treatment for 50% of Caucasians with MUM. Studies to date have shown that survival in patients receiving Tebentafusp-tebn was prolonged by approximately 6 months compared to standard care treatments. But the response among patients who receive Tebentafusp has been low and stands at approximately 9%. A patient’s response to immunotherapy is typically evaluated by assessing tumor size through imaging. Imaging based evaluation has its own challenges because of confounding factors including atypical patterns, pseudoprogression, hyper-progression, and delayed response. Molecular markers that can gauge sensitively and specifically the response to immunotherapy, and the speed of that response, could drastically improve the quality of clinical decision making.
Several molecular determinants of immunotherapy response have been explored to date, including liver and serological RNAs, protein biomarkers, or circulating tumor DNA, and are found to lack in either sensitivity or specificity. In this project, we will explore the development of biomarkers of response to immunotherapy that are based on short non-coding RNAs (sncRNAs). To the best of our knowledge, there have been no previous attempts to develop such sncRNA-based biomarkers. SncRNAs are made by cells simultaneously with proteins but are not used to make proteins. Instead, they regulate the abundance of proteins, and, by extension, cell fate. These roles make sncRNAs very important. Indeed, we now know that sncRNAs control many essential cellular processes, in health and disease. Advances during the last decade have demonstrated that the levels of sncRNAs and their control on proteins change depending on tissue type and disease type. This has led to the development of sensitive and specific diagnostic and prognostic tools that leverage these changes. It is these attributes of sncRNAs that led us to choose them as the basis for this project.
The project has two parts. In the first part, we will study liver tumors from MUM patients participating in the phase I/II trial at Jefferson. We will examine the levels of (a) sncRNAs and (b) the RNA templates used to make proteins in these tumors. We will determine how these levels differ from what is seen in the primary tumors of UM patients who developed or did not develop metastases. We will also determine how these levels change over time during immunotherapy treatment. In the second part, we will study sncRNAs in the blood of the same patients at different time points during treatment. We will determine how their levels differ from those in the blood of UM patients without metastases. We will also determine how these levels change over time as treatment progresses. Since we will collect many more datapoints by profiling patient blood, we expect also to build a mathematical model that captures the speed of response to immunotherapy. This model will help us assess the response of future patients receiving the same therapy as they undergo therapy. We also note that if there are enough responders and non-responders among the patients participating in the trial, we will explore developing a prognostic biomarker of response that will be based on the sncRNA profiles obtained from either the biopsies or blood.
Our short-term goal is to create biomarkers that leverage the granular description of cellular processes captured by sncRNAs. If we are successful, we expect the biomarkers will be used in clinical settings to assist in clinical decision-making. Our long-term goal is to extend patient survival by creating novel targeted therapies for MUM. Lastly, we stress that the analytical methods we will develop are very general in nature. As such they can be used in conjunction with other therapeutic regimens where it is important to develop sensitive and specific molecular tests for determining response to therapy.
Melanoma of the eye, also known as uveal melanoma (UM), is a rare tumor that originates from pigment producing cells (melanocytes) in the eye. Compared to cutaneous (skin) melanoma UM is rare, yet it is the most common primary intraocular malignancy in adults. During the past decade, substantial progress has been achieved in the treatment of primary eye lesions that allow for the preservation of the affected eye. However, nearly one half of UM patients progress to develop metastases that most often localize to the liver. Rapid tumor progression in the liver leads to death within 4 to 15 months. The use of chemotherapy has proven of little value to date because metastatic UM (MUM) is highly resistant to it. Consequently, during the last decade, researchers have been investigating an alternative form of therapy called immunotherapy.
Immunotherapy works differently than chemotherapy. Here, the patient’s immune system is activated in a targeted manner to attack cancer cells. Immunotherapy has proven very powerful in numerous contexts and several drugs (“immune check point inhibitors”) have already been approved by the US Food and Drug Administration. Immune check point inhibitors greatly improved the overall survival of skin melanoma patients but have shown little benefit for UM patients. However, a new FDA-approved therapy for UM, Tebentafusp-tebn, is more promising. The therapy works by increasing the numbers of cancer-fighting cells and is currently the preferred treatment for 50% of Caucasians with MUM. Studies to date have shown that survival in patients receiving Tebentafusp-tebn was prolonged by approximately 6 months compared to standard care treatments. But the response among patients who receive Tebentafusp has been low and stands at approximately 9%. A patient’s response to immunotherapy is typically evaluated by assessing tumor size through imaging. Imaging based evaluation has its own challenges because of confounding factors including atypical patterns, pseudoprogression, hyper-progression, and delayed response. Molecular markers that can gauge sensitively and specifically the response to immunotherapy, and the speed of that response, could drastically improve the quality of clinical decision making.
Several molecular determinants of immunotherapy response have been explored to date, including liver and serological RNAs, protein biomarkers, or circulating tumor DNA, and are found to lack in either sensitivity or specificity. In this project, we will explore the development of biomarkers of response to immunotherapy that are based on short non-coding RNAs (sncRNAs). To the best of our knowledge, there have been no previous attempts to develop such sncRNA-based biomarkers. SncRNAs are made by cells simultaneously with proteins but are not used to make proteins. Instead, they regulate the abundance of proteins, and, by extension, cell fate. These roles make sncRNAs very important. Indeed, we now know that sncRNAs control many essential cellular processes, in health and disease. Advances during the last decade have demonstrated that the levels of sncRNAs and their control on proteins change depending on tissue type and disease type. This has led to the development of sensitive and specific diagnostic and prognostic tools that leverage these changes. It is these attributes of sncRNAs that led us to choose them as the basis for this project.
The project has two parts. In the first part, we will study liver tumors from MUM patients participating in the phase I/II trial at Jefferson. We will examine the levels of (a) sncRNAs and (b) the RNA templates used to make proteins in these tumors. We will determine how these levels differ from what is seen in the primary tumors of UM patients who developed or did not develop metastases. We will also determine how these levels change over time during immunotherapy treatment. In the second part, we will study sncRNAs in the blood of the same patients at different time points during treatment. We will determine how their levels differ from those in the blood of UM patients without metastases. We will also determine how these levels change over time as treatment progresses. Since we will collect many more datapoints by profiling patient blood, we expect also to build a mathematical model that captures the speed of response to immunotherapy. This model will help us assess the response of future patients receiving the same therapy as they undergo therapy. We also note that if there are enough responders and non-responders among the patients participating in the trial, we will explore developing a prognostic biomarker of response that will be based on the sncRNA profiles obtained from either the biopsies or blood.
Our short-term goal is to create biomarkers that leverage the granular description of cellular processes captured by sncRNAs. If we are successful, we expect the biomarkers will be used in clinical settings to assist in clinical decision-making. Our long-term goal is to extend patient survival by creating novel targeted therapies for MUM. Lastly, we stress that the analytical methods we will develop are very general in nature. As such they can be used in conjunction with other therapeutic regimens where it is important to develop sensitive and specific molecular tests for determining response to therapy.


